Feature & Interview on Escobedo’s Research
Escobedo’s group research is centered on the development and application of simulation and modeling methods to elucidate the connection between microscopic structure and macroscopic properties of polymeric and colloidal materials. The ultimate goal of generating such new fundamental knowledge is to improve the engineering of materials of desirable or “super” properties that originate in the creation of special types of structural order or the control of phase transitions. Our work has in particular focused on elucidating the role of entropy as an important (and often overlooked) force that can be harnessed to help us create materials with desirable properties. Entropic forces are often crucial in the formation of materials whose molecular order is intermediate between crystals (having perfect order and reduced entropy) and liquids (having high degree of disorder and high entropy), such as liquid crystals, plastic solids, elastomers, gels, microsegregated phases of block copolymers, and biomolecules with order domains like proteins. Such intermediate order is often manifested in the form of phases with novel structures and a combination of physical properties not observed in common materials. These mesophases are hence attractive for such new uses as nanoporous materials for active layers in solar cells, membranes for ultra-filtration, light amplifiers and optic guides for lasers, liquid armor, plastics of high elasticity and toughness, and new therapeutic antibodies. The figure below illustrates some of the systems of interest.
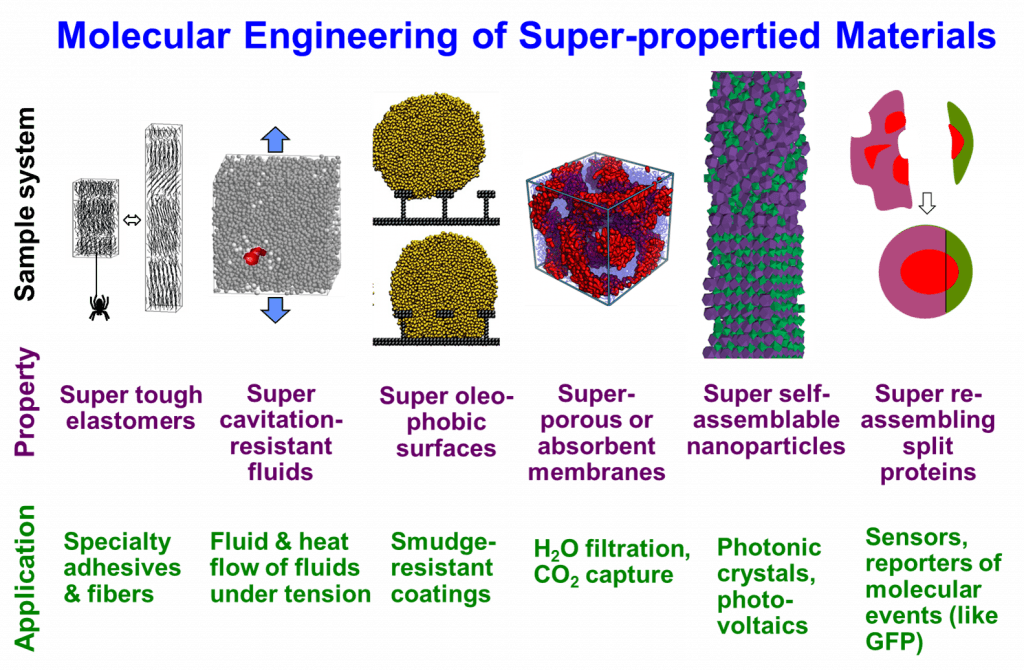
Entropy, in contrast to energy, is more difficult to appreciate and quantify, even though nature exploits the interplay between energetic and entropic forces to determine the “final solution”; i.e., the physical behavior of any material and the biological function of any biomolecule. In this context, the rational design of materials and molecules with “super” properties can only be possible if we can control entropy. Although entropy is usually associated with disorder, we have demonstrated that different types of entropy can be manipulated to give rise to a wide variety of structural order. In stabilizing a sought-after phase, entropy sometimes opposes energy (e.g., the increase of conformational entropy of a homopolymer additive allows diblock copolymers to form bicontinuous phases by countering the increase of interfacial energy), but sometimes entropy can help energy (e.g., in our proposed super-tough elastomers that rely on multiple order-disordered transitions: the deximing tendency between chain blocks of different backbone stiffness is coupled with the segregation tendency of chain blocks with unfavorable energetic interactions). Moreover, some phases result from the competition of different types of entropy; e.g., colloidal particles of polyhedral shape may adopt partial or complete order depending on whether orientational entropy or packing entropy prevails. Ultimately, entropy should be seen as a very “creative” force in nature and many of our studies are examples of doing “engineering” with entropy to create new types of microscopic order that manifest in desirable material properties and functions.




